|
产品名称 |
U-937人组织细胞淋巴瘤细胞 |
|
货号 |
ZQ0087 |
|
产品介绍 |
U-937是一种人组织细胞淋巴瘤细胞系,最初由Sundstrom和Nilsson在1974年从一位37岁白人男性患者细胞淋巴瘤病人的胸腔积液中分离得到。这种细胞系具有单核细胞形态,能在悬浮状态下生长,并且可以通过特定的诱导剂如人混合淋巴细胞培养上清、佛波脂、维生素D3、γ干扰素、肿瘤坏死因子和维甲酸等诱导终末单核细胞分化。该细胞免疫球蛋白产物和EB病毒表达为阴性。该细胞表达federation of american scientists 美国科学家联合会抗原且对TNF和抗-Fas抗体敏感。 |
|
种属 |
人 |
|
性别/年龄 |
男/37岁 |
|
组织 |
胸膜/胸腔积液,淋巴细胞,髓样 |
|
疾病 |
组织细胞淋巴瘤 |
|
细胞类型 |
肿瘤细胞 |
|
形态学 |
单核细胞 |
|
生长方式 |
悬浮 |
|
倍增时间 |
大约30~100小时 |
|
培养基和添加剂 |
RPMI-1640(中乔新舟 货号:ZQ-200)+10%胎牛血清(中乔新舟 货号:ZQ0500)+1%双抗(中乔新舟 货号:CSP006) |
|
推荐完全培养基货号 |
|
|
生物安全等级 |
BSL-1 |
|
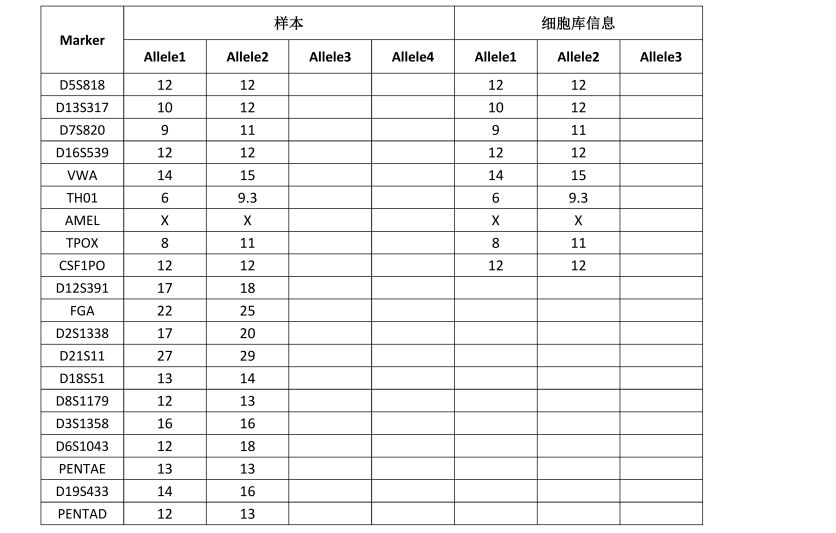
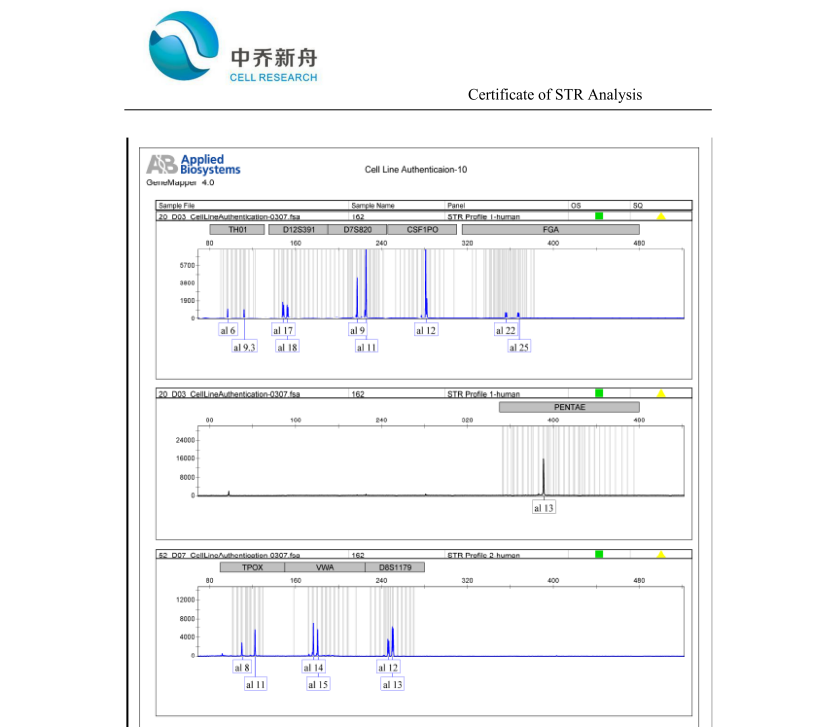
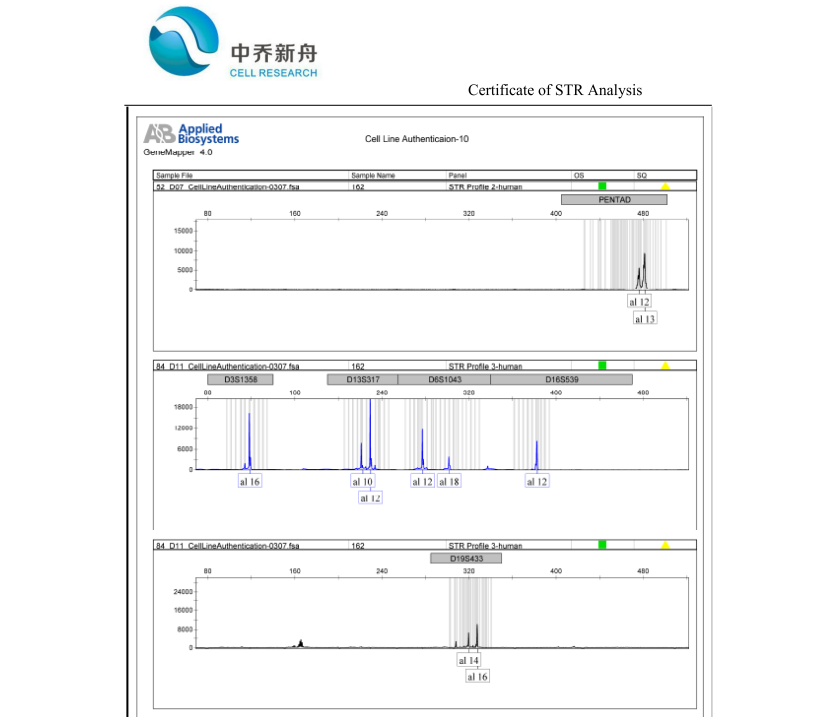
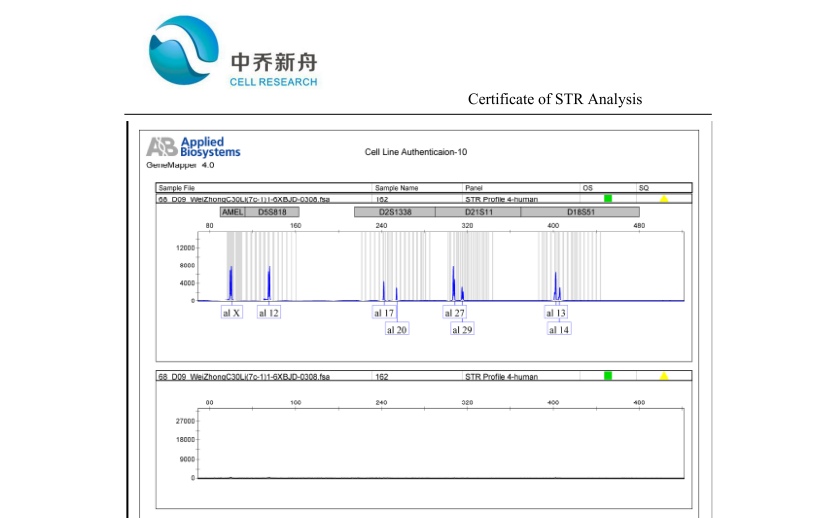
STR位点信息 |
Amelogenin: X
CSF1PO: 12
|
|
培养条件 |
95%空气,5%二氧化碳;37℃ |
|
抗原表达/受体表达 |
*** |
|
基因表达 |
*** |
|
保藏机构 |
ATCC; CRL-1593 ATCC; CRL-1593.2 DSMZ; ACC-5 ECACC; 85011440 |
|
供应限制 |
仅供科研使用 |
|
货号 |
ZQ0087 |
|
发货规格 |
活细胞:T25培养瓶*1瓶或者1ml 冻存管*2支(细胞量约为1x10^6 Cells/Vial)二选一 |
|
发货形式 |
活细胞:常温运输;冻存管:干冰运输 |
|
储存温度 |
活细胞:培养箱;冻存管:液氮罐 |
|
产地 |
中国 |
|
供应限制 |
仅供科研使用 |
原文链接: https://www.sciencedirect.com/science/article/pii/S0024320519311567
2、PubMed=16311011; DOI=10.1016/j.tiv.2005.10.012
Ashikaga T., Yoshida Y., Hirota M., Yoneyama K., Itagaki H., Sakaguchi H., Miyazawa M., Ito Y., Suzuki H., Toyoda H.
Development of an in vitro skin sensitization test using human cell lines: the human Cell Line Activation Test (h-CLAT). I. Optimization of the h-CLAT protocol.
Toxicol. In Vitro 20:767-773(2006)
3、PubMed=16337770; DOI=10.1016/j.tiv.2005.10.014
Sakaguchi H., Ashikaga T., Miyazawa M., Yoshida Y., Ito Y., Yoneyama K., Hirota M., Itagaki H., Toyoda H., Suzuki H.
Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT). II. An inter-laboratory study of the h-CLAT.
Toxicol. In Vitro 20:774-784(2006)
4、PubMed=16408098; DOI=10.1038/sj.leu.2404081
Quentmeier H., MacLeod R.A.F., Zaborski M., Drexler H.G.
JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders.
Leukemia 20:471-476(2006)
5、PubMed=20801507; DOI=10.1016/j.leukres.2010.07.040
Minafra L., Di Cara G., Albanese N.N., Cancemi P.
Proteomic differentiation pattern in the U937 cell line.
Leuk. Res. 35:226-236(2011)
6、PubMed=21269460; DOI=10.1186/1752-0509-5-17
Burkard T.R., Planyavsky M., Kaupe I., Breitwieser F.P., Burckstummer T., Bennett K.L., Superti-Furga G., Colinge J.
Initial characterization of the human central proteome.
BMC Syst. Biol. 5:17.1-17.13(2011)
7、PubMed=22460905; DOI=10.1038/nature11003
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
8、DOI=10.1016/j.actaastro.2013.06.007
Paulsen K., Tauber S., Golz N., Simmet D.M., Engeli S., Birlem M., Dumrese C., Karer A., Hunziker S., Biskup J., Konopasek S., Suh D., Breuer-Hurlimann E., Signer C., Wang A., Sang C., Grote K.-H., Zhuang F.-Y., Ullrich O.
Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity -- results of an in vitro experiment on board of the Shenzhou-8 space mission.
Acta Astronaut. 94:277-292(2013)
9、PubMed=25485619; DOI=10.1038/nbt.3080
Klijn C., Durinck S., Stawiski E.W., Haverty P.M., Jiang Z.-S., Liu H.-B., Degenhardt J., Mayba O., Gnad F., Liu J.-F., Pau G., Reeder J., Cao Y., Mukhyala K., Selvaraj S.K., Yu M.-M., Zynda G.J., Brauer M.J., Wu T.D., Gentleman R.C., Manning G., Yauch R.L., Bourgon R., Stokoe D., Modrusan Z., Neve R.M., de Sauvage F.J., Settleman J., Seshagiri S., Zhang Z.-M.
A comprehensive transcriptional portrait of human cancer cell lines.
Nat. Biotechnol. 33:306-312(2015)
10、PubMed=25877200; DOI=10.1038/nature14397
Yu M., Selvaraj S.K., Liang-Chu M.M.Y., Aghajani S., Busse M., Yuan J., Lee G., Peale F.V., Klijn C., Bourgon R., Kaminker J.S., Neve R.M.
A resource for cell line authentication, annotation and quality control.
Nature 520:307-311(2015)
11、PubMed=26589293; DOI=10.1186/s13073-015-0240-5
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
12、PubMed=29787063; DOI=10.1007/978-3-319-16104-4_14
Chanput W., Peters V., Wichers H.J.
THP-1 and U937 cells.
(In) The impact of food bioactives on health. In vitro and ex vivo models; Verhoeckx K., Cotter P., Lopez-Exposito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H. (eds.); pp.147-159; Springer; Cham (2015)
13、PubMed=30285677; DOI=10.1186/s12885-018-4840-5
Tan K.-T., Ding L.-W., Sun Q.-Y., Lao Z.-T., Chien W., Ren X., Xiao J.-F., Loh X.-Y., Xu L., Lill M., Mayakonda A., Lin D.-C., Yang H., Koeffler H.P.
Profiling the B/T cell receptor repertoire of lymphocyte derived cell lines.
BMC Cancer 18:940.1-940.13(2018)
14、PubMed=30629668; DOI=10.1371/journal.pone.0210404
Uphoff C.C., Pommerenke C., Denkmann S.A., Drexler H.G.
Screening human cell lines for viral infections applying RNA-Seq data analysis.
PLoS ONE 14:E0210404-E0210404(2019)
15、PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)
16、PubMed=31068700; DOI=10.1038/s41586-019-1186-3
Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R. III, Barretina J.G., Gelfand E.T., Bielski C.M., Li H.-X., Hu K., Andreev-Drakhlin A.Y., Kim J., Hess J.M., Haas B.J., Aguet F., Weir B.A., Rothberg M.V., Paolella B.R., Lawrence M.S., Akbani R., Lu Y.-L., Tiv H.L., Gokhale P.C., de Weck A., Mansour A.A., Oh C., Shih J., Hadi K., Rosen Y., Bistline J., Venkatesan K., Reddy A., Sonkin D., Liu M., Lehar J., Korn J.M., Porter D.A., Jones M.D., Golji J., Caponigro G., Taylor J.E., Dunning C.M., Creech A.L., Warren A.C., McFarland J.M., Zamanighomi M., Kauffmann A., Stransky N., Imielinski M., Maruvka Y.E., Cherniack A.D., Tsherniak A., Vazquez F., Jaffe J.D., Lane A.A., Weinstock D.M., Johannessen C.M., Morrissey M.P., Stegmeier F., Schlegel R., Hahn W.C., Getz G., Mills G.B., Boehm J.S., Golub T.R., Garraway L.A., Sellers W.R.
Next-generation characterization of the Cancer Cell Line Encyclopedia.
Nature 569:503-508(2019)
17、PubMed=31160637; DOI=10.1038/s41598-019-44491-x
Quentmeier H., Pommerenke C., Dirks W.G., Eberth S., Koeppel M., MacLeod R.A.F., Nagel S., Steube K., Uphoff C.C., Drexler H.G.
The LL-100 panel: 100 cell lines for blood cancer studies.
Sci. Rep. 9:8218-8218(2019)
18、PubMed=31978347; DOI=10.1016/j.cell.2019.12.023
Nusinow D.P., Szpyt J., Ghandi M., Rose C.M., McDonald E.R. III, Kalocsay M., Jane-Valbuena J., Gelfand E.T., Schweppe D.K., Jedrychowski M.P., Golji J., Porter D.A., Rejtar T., Wang Y.K., Kryukov G.V., Stegmeier F., Erickson B.K., Garraway L.A., Sellers W.R., Gygi S.P.
Quantitative proteomics of the Cancer Cell Line Encyclopedia.
Cell 180:387-402.e16(2020)
19、PubMed=33317567; DOI=10.1186/s13039-020-00517-y
MacKinnon R.N., Peverall J., Campbell L.J., Wall M.
Detailed molecular cytogenetic characterisation of the myeloid cell line U937 reveals the fate of homologous chromosomes and shows that centromere capture is a feature of genome instability.
Mol. Cytogenet. 13:50.1-50.14(2020)
20、PubMed=34707142; DOI=10.1038/s41598-021-00623-w
Maher M., Diesch J., Le Pannerer M.-M., Cabezon M., Mallo M., Vergara S., Mendez Lopez A., Mesa Tudel A., Sole F., Sorigue M., Zamora L., Granada I., Buschbeck M.
Divergent leukaemia subclones as cellular models for testing vulnerabilities associated with gains in chromosomes 7, 8 or 18.
Sci. Rep. 11:21145-21145(2021)
| 产品名称 | 价格 | 指令 |
| Raji人Burkitts淋巴瘤细胞(STR鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| EL-4小鼠淋巴瘤细胞(种属鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| EB-3人淋巴样Burkitt's淋巴瘤细胞(STR鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| YAC-1小鼠淋巴瘤细胞(种属鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| L5178Y TK+/- clone (3.7.2C)小鼠淋巴瘤细胞(种属鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| Daudi人Burkitts淋巴瘤细胞(STR鉴定)[细胞+500ml专培套餐促销] | ¥980.00 | 购物车 》 |
| JeKo-1人套细胞淋巴瘤细胞 (STR鉴定) | ¥1800.00 | 购物车 》 |
| 支原体清除试剂盒 | ¥600.00 | 购物车 》 |
| 国产转染试剂 | ¥800.00 | 购物车 》 |
| 慢病毒介导基因沉默或过表达 | ¥询价 | 购物车 》 |
| RPMI-1640基础培养基 | ¥56.00 | 购物车 》 |
| RPMI-1640完全培养基(10%FBS) | ¥350.00 | 购物车 》 |
| 优级胎牛血清 | ¥2580(开学促销) | 购物车 》 |
| 人源细胞STR鉴定服务 | ¥900.00 | 购物车 》 |
 上海中乔新舟生物科技有限公司
上海中乔新舟生物科技有限公司